Which compound contains a triple bond in its Lewis structure?
A) CO
B) SO2
C) H2S
D) 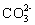
Correct Answer:
Verified
Q65: Which does not represent the correct formula
Q66: Which atom has the largest radius?
A)Se
B)Te
C)O
D)S
Q67: The shape of a carbon tetrachloride molecule
Q68: In the following molecular shape,the angle is
Q69: Which atom has four valence electrons?
A)Be
B)F
C)Ge
D)Te
Q71: Which does not have a noble gas
Q72: The total number of valence electrons in
Q73: Carbon dioxide is a nonpolar molecule because
A)oxygen
Q74: Ionic bonds will most likely form between
A)two
Q75: The molecular shape shown in the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents