The molecular shape shown in the following figure is 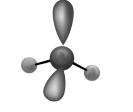
A) tetrahedral.
B) trigonal pyramidal.
C) bent.
D) trigonal planar.
Correct Answer:
Verified
Q70: Which compound contains a triple bond in
Q71: Which does not have a noble gas
Q72: The total number of valence electrons in
Q73: Carbon dioxide is a nonpolar molecule because
A)oxygen
Q74: Ionic bonds will most likely form between
A)two
Q76: Which series is ranked in order of
Q77: To break a covalent bond,energy must be
A)released.
B)absorbed.
C)both,absorbed
Q78: Which compound has double covalent bonds within
Q79: Which atom has the largest radius?
A)Mg
B)Si
C)S
D)Cl
Q80: Which of the following does not contain
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents