A sample of ice at 0° C absorbs 6030 J of heat energy.How much of the ice can melt? The heat of fusion of ice is 335 J/g.
A) 6030 g
B) 2.02 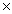 10 6 g
10 6 g
C) 18.0 g
D) 5700 g
Correct Answer:
Verified
Q75: Hydrogen bonding
A)occurs only between water molecules.
B)is stronger
Q76: A hydrogen bond is
A)a covalent bond between
Q77: What quantity of heat is required to
Q78: What quantity of heat is required to
Q79: What quantity of heat is required to
Q81: Small amounts of water evaporate at room
Q82: Evaporation is an exothermic process.
Q83: When a piece of metallic copper is
Q84: Write the balanced chemical equations for the
Q85: Water purification often involves screening,flocculation and sedimentation,sand
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents