In which direction will the point of equilibrium shift when pressure is increased in the following equilibrium? KCl(s) 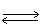 K+1 (aq) + Cl-1 (aq)
K+1 (aq) + Cl-1 (aq)
A) Shift to the right
B) Shift to the left
C) No shift
Correct Answer:
Verified
Q13: According to Le Châtelier's Principle,decreasing the temperature
Q14: In which direction will the point of
Q15: Equilibrium is reached in a chemical reaction
Q16: In which direction will the point of
Q17: In which direction will the point of
Q19: The main reason for the increase in
Q20: In which direction will the point of
Q21: Which acid ionization constant would indicate the
Q22: In the following equilibrium;as I2 (g)is added,the
Q23: Calculate the value of Keq for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents