In the following equilibrium;as I2 (g) is added,the concentration of H2 (g) will H2 (g) + I2 (g) 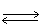 2 HI (g)
2 HI (g)
A) increase.
B) decrease.
C) remain the same.
Correct Answer:
Verified
Q17: In which direction will the point of
Q18: In which direction will the point of
Q19: The main reason for the increase in
Q20: In which direction will the point of
Q21: Which acid ionization constant would indicate the
Q23: Calculate the value of Keq for the
Q24: Which solubility product constant indicates the least
Q25: What is the [OH-1] in a 0.01
Q26: Which solubility product constant indicates the most
Q27: Which Keq value indicates the greatest concentration
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents