Multiple Choice
In the following equilibrium,as HNO3 is added,the concentration of CrO4-2 ion will 2 CrO4-2 (aq) + 2 H+1 (aq) 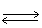 Cr2O7-2 (aq) + H2O (l)
Cr2O7-2 (aq) + H2O (l)
A) increase.
B) decrease.
C) remain the same.
Correct Answer:
Verified
Related Questions
Q29: What is the [OH-1] in a 0.0001
Q30: Which acid ionization constant would indicate the
Q31: Which Keq value indicates the greatest concentration
Q32: What is the [H+1] in a 0.01
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents