For the following reaction: 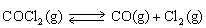 Keq =
Keq = 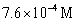 .COCl2 is introduced in a closed container until its concentration is 0.890 M.What will be the concentration of Cl2(g) once the reaction has reached equilibrium?
.COCl2 is introduced in a closed container until its concentration is 0.890 M.What will be the concentration of Cl2(g) once the reaction has reached equilibrium?
A) 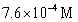
B) 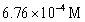
C) 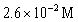
D) 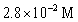
Correct Answer:
Verified
Q28: In the following equilibrium,as volume of the
Q29: What is the [OH-1] in a 0.0001
Q30: Which acid ionization constant would indicate the
Q31: Which Keq value indicates the greatest concentration
Q32: What is the [H+1] in a 0.01
Q34: In the following equilibrium,as HNO3 is added,the
Q35: An aqueous solution of sodium sulfate will
Q36: In the following equilibrium;as H2 (g)is removed,the
Q37: In the following equilibrium,as the concentration of
Q38: In the following equilibrium,as temperature increases,the point
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents