In a closed reaction vessel having a volume of 1.00 L,1.25g of N2O4 are allowed to undergo the following reaction.
N2O4 (g) 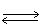 2 NO2 (g)
2 NO2 (g) 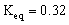 What will be the equilibrium concentrations of NO2(g)and N2O4(g)in moles/Liter?
What will be the equilibrium concentrations of NO2(g)and N2O4(g)in moles/Liter?
Correct Answer:
Verified
Q89: A 7.5 L vessel contains 10.0g of
Q90: Aqueous solutions of NaBr,K2SO4,and NaF will be
Q91: In a closed reaction vessel the following
Q92: The Ksp for silver bromide is 5.0
Q93: An aqueous solution containing both Na3PO4 and
Q95: If the Ka of acetic acid is
Q96: When aqueous nitrous acid is completely neutralized
Q97: The ionization constant,Ka,for an acetic acid solution
Q98: The acid ionization constant,Ka,of hydrosulfuric acid is
Q99: The Ksp of lead(II)chloride is 1.6
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents