A 7.5 L vessel contains 10.0g of NO2 (g)and 0.55g of N2O4 (g) at equilibrium,as follows
2 NO2 (g) 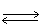 N2O4 (g)
N2O4 (g)
Calculate the value of the equilibrium constant for this reaction.
Correct Answer:
Verified
Q84: The Ksp value for copper(II)sulfide is 8.5
Q85: The presence of a catalyst will increase
Q86: Adding more solid silver chloride to a
Q87: The addition of a catalyst will change
Q88: A solution of sodium acetate in water
Q90: Aqueous solutions of NaBr,K2SO4,and NaF will be
Q91: In a closed reaction vessel the following
Q92: The Ksp for silver bromide is 5.0
Q93: An aqueous solution containing both Na3PO4 and
Q94: In a closed reaction vessel having a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents