The Ksp value for copper(II)sulfide is 8.5 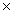 10-45.Calculate the solubility of copper(II) sulfide in grams per liter.
10-45.Calculate the solubility of copper(II) sulfide in grams per liter.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q79: If HCl (aq)is added to a saturated
Q80: A 0.30 M solution of formic acid,HCHO2,is
Q81: The value of the equilibrium constant is
Q82: The Ksp value for barium sulfate is
Q83: The acid ionization constant,Ka,for ammonium chloride is
Q85: The presence of a catalyst will increase
Q86: Adding more solid silver chloride to a
Q87: The addition of a catalyst will change
Q88: A solution of sodium acetate in water
Q89: A 7.5 L vessel contains 10.0g of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents