Adding more solid silver chloride to a saturated aqueous solution of silver chloride will shift the equilibrium to the right. 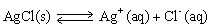
Correct Answer:
Verified
Q81: The value of the equilibrium constant is
Q82: The Ksp value for barium sulfate is
Q83: The acid ionization constant,Ka,for ammonium chloride is
Q84: The Ksp value for copper(II)sulfide is 8.5
Q85: The presence of a catalyst will increase
Q87: The addition of a catalyst will change
Q88: A solution of sodium acetate in water
Q89: A 7.5 L vessel contains 10.0g of
Q90: Aqueous solutions of NaBr,K2SO4,and NaF will be
Q91: In a closed reaction vessel the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents