Aspirin (C9H8O4) is produced by the reaction of salicylic acid (M = 138.1 g/mol) and acetic anhydride
(M = 102.1 g/mol) .C7H6O3(s) + C4H6O3( 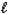 ) C9H8O4(s) + C2H4O2(
) C9H8O4(s) + C2H4O2( 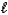
)
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol) can theoretically be obtained?
A) 0.40 g
B) 2.61 g
C) 2.82 g
D) 1.53 g
E) 3.60 g
Correct Answer:
Verified
Q14: How many moles of Mg3P2(s)can be
Q15: The compound P4S3 is used in
Q16: Calculate the number of moles of O2
Q17: If the complete combustion of an
Q18: Potassium hydrogen carbonate decomposes with heat
Q20: One step in the isolation of pure
Q21: Aspirin is produced by the reaction
Q22: Complete combustion of a 0.30-mol sample of
Q24: Of the following,the only empirical formula is
A)
Q36: A 1.850 g mixture of SrCO3 and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents