Multiple Choice
Consider the reaction: 2 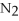
O(g) ⇌ 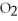
(g) + 2 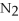
(g) .Which of the following will cause a shift in the equilibrium to the left?
1.Remove 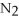
O
2.Remove 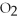
3.Add 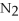
A) 1 and 2 only
B) 1 and 3 only
C) 2 and 3 only
D) All of 1,2,and 3
E) Neither 1,2,or 3
Correct Answer:
Verified
Related Questions
Q81: What happens to the equilibrium position of
Q90: Which compound below is the most soluble
Q91: For the reaction H2 (g)+ Cl2 (g)⇌

