Given the choice of adding ions of Mg 2+ (aq) ,Ca 2+ (aq) ,Sr 2+ (aq) or Ba 2+ (aq) to a solution of carbonate ions,CO3 2-,which choice would precipitate an insoluble carbonate compound first?
Use the following 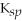
Data:
MgCO3 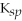
= 6.82 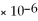
CaCO3 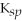
= 4.96 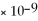
SrCO3 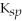
= 9.30 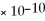
BaCO3 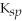
= 5.00 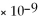
A) Mg 2+
B) Ca 2+
C) Sr 2+
D) Ba 2+
E) none of these will give a precipitate
Correct Answer:
Verified
Q81: What happens to the equilibrium position of
Q91: For the reaction H2 (g)+ Cl2 (g)⇌
Q95: Consider the reaction: 2 Q96: Consider the reaction: 2 Q100: We have the following reaction at equilibrium Q100: What is the molar solubility, (S),of FeS Q102: Calculate the equilibrium constant (Keq)for the reaction Q104: What is the molar solubility, (S),of FeCO3 Q107: Which statement below is FALSE? Q108: The effect of a catalyst is to:![]()
![]()
A)The rate of
A)increase
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents