Calculate the equilibrium constant for the spontaneous redox reaction at 298 K that would occur using the following two half-reactions.
ClO3-(aq) + 6 H+(aq) + 5 e- 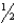 Cl2(g) + 3 H2O(
Cl2(g) + 3 H2O( 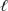 ) E° = +1.47 V
) E° = +1.47 V
HClO(aq) + H+(aq) + e- 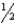 Cl2(s) + H2O(
Cl2(s) + H2O( 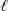 )
)
E° = +1.63 V
A) 5.04 102
B) 1.98 10-3
C) 2.32 1052
D) 0.996
E) 3.26 1013
Correct Answer:
Verified
Q35: The unit of electrical power, watt (W),
Q73: Which one of the following is NOT
Q75: The unit of current, the ampere (A),
Q106: The electrodes on batteries are labeled +
Q107: Bubbles will form on wires attached to
Q107: The charge supplied by a battery can
Q108: Electrochemical cell potentials can be used
Q109: Suppose the redox reaction in a
Q115: A NiMH battery uses _ as the
Q115: The average electrical current delivered if
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents