For the electrochemical cell based on the reaction of zinc metal with copper(II)ions indicated below where zinc is being oxidized,identify the components labeled as a-e.The anode is on the left; the cathode,on the right.Use the terms zinc nitrate solution,copper nitrate solution,copper electrode,zinc electrode,and salt bridge.Also,label the electrodes as + and -. 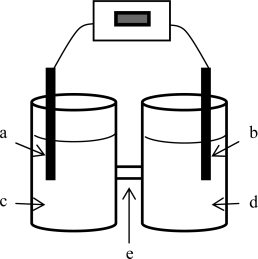
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q138: Which of A-D is a disadvantage in
Q139: Methanol fuel cells depend on the
Q140: The oxidation of hydrogen by oxygen
Q141: A 1.5 V alkaline battery is constructed
Q142: Write the cell diagram for the
Q144: How many coulombs of charge are
Q145: What is the cell potential for a
Q147: What is the standard cell potential
Q148: What constitutes a standard hydrogen electrode?
Q148: Silver tarnishes due to the formation of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents