Based on the information in the table of standard reduction potentials below,explain what,if anything,will happen if a piece of solid lead is placed in an aqueous solution containing gold(III)ions.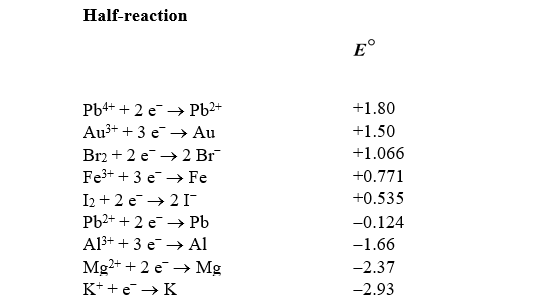
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q108: Which statement regarding battery-powered electric cars is
Q115: When a reduction half-reaction is written as
Q134: What must be true about the
Q145: What is the cell potential for a
Q147: What is the standard cell potential
Q148: Silver tarnishes due to the formation of
Q148: What constitutes a standard hydrogen electrode?
Q151: Write the cell diagram for the
Q152: Ozone can react with manganese(II)ions in
Q153: Based on the information in the table
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents