Because of recent advances in recovery technology,the supply of natural gas,which is mostly methane,has increased significantly.Suppose a fuel cell based on the oxidation of methane by oxygen to produce carbon dioxide and water is designed and constructed for use in automobile engines.Estimate the maxium amount of work that can be produced by the electric motor when 321 grams of methane (16.05 g/mol)is consumed.The data in the table below may be helpful.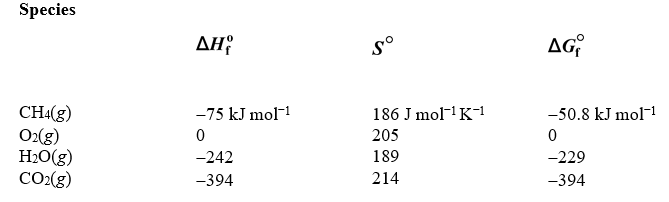
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q151: What is the most important use for
Q161: How does a fuel cell differ from
Q165: Estimate the value of Kf for
Q166: Because of recent advances in recovery technology,the
Q167: Permanganate ions can oxidize sulfite in
Q169: If,in using a lead-acid battery to start
Q170: A concentration cell is made using Ni2+
Q171: Suppose a NiMH battery is rated
Q172: Chromium often is electroplated on other metals
Q174: Estimate the value of Ksp for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents