Arrange the following in order of increasing boiling point: (a)dimethyl ether; (b)n-propane; (c)ethylene oxide; and (d)acetaldehyde (shown in order).
a. 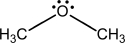
b. 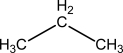
c. 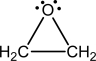
d. 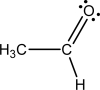
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q60: At the triple point of a substance,
A)three
Q80: Which of the following statements is false
Q83: Many substances are quite soluble in water.Identify
Q85: Difluoromethane (CH2F2)has a dipole moment of 1.93
Q86: Sketch the hydrated ions that form when
Q86: The boiling points of group IVA hydrides
Q87: For each of the following pairs of
Q88: Briefly explain the following observations using intermolecular
Q89: For each of the following pairs of
Q160: Why do the strengths of dispersion interactions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents