What is the value of the reaction quotient,Q,for the voltaic cell constructed from the following two half-reactions when the Zn2+ concentration is 0.0103 M and the Ag+ concentration is 1.35 M? Zn2+(aq) + 2e- Zn(s) ; ° = -0.76 V
Ag+(aq) + e- Ag(s) ; ° = 0.80 V
A) 177
B) 131
C) 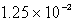
D) 
E) 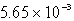
Correct Answer:
Verified
Q110: For the cell Cu(s)| Cu2+ || Ag+
Q111: A common car battery consists of
Q112: An antique automobile bumper is to be
Q113: Electrolysis of a molten salt with the
Q114: What is the potential at 25°C
Q116: Copper is electroplated from CuSO4 solution.A
Q117: Which of the following statements is
Q118: Which of the following statements about batteries
Q119: In order to determine the identity
Q120: Concentration cells work because standard reduction potentials
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents