A common car battery consists of six identical cells each of which carries out the reaction: Pb + PbO2 + 2HSO4- + 2H+ 2PbSO4 + 2H2O
Suppose that in starting a car on a cold morning a current of 125 amperes is drawn for 18.4 seconds from a cell of the type described above.How many grams of Pb would be consumed? (The atomic weight of Pb is 207.19. )
A) 4.94 g
B) 2.47 g
C) 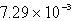 g
g
D) 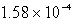 g
g
E) 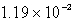 g
g
Correct Answer:
Verified
Q106: Which of the following statements about corrosion
Q107: What quantity of charge is required to
Q108: What is
Q109: How many moles of electrons are
Q110: For the cell Cu(s)| Cu2+ || Ag+
Q112: An antique automobile bumper is to be
Q113: Electrolysis of a molten salt with the
Q114: What is the potential at 25°C
Q115: What is the value of the
Q116: Copper is electroplated from CuSO4 solution.A
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents