How many seconds would it take to deposit 18.2 g of Ag (atomic mass = 107.87) from a solution of AgNO3 using a current of 10.00 amp?
A) 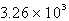 s
s
B) 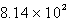 s
s
C) 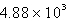 s
s
D) 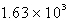 s
s
E) 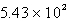 s
s
Correct Answer:
Verified
Q128: An unknown metal (M)is electrolyzed.It took 74.1
Q129: A solution of MnO42- is electrolytically reduced
Q130: Consider a galvanic cell with a
Q131: Nickel is electroplated from a NiSO4 solution.A
Q132: Gold (atomic mass = 197.0)is plated from
Q133: What mass of chromium could be deposited
Q134: What are the products of the chlor-alkali
Q135: Which of the following used to be
Q136: Balance the following equation: Zn +
Q137: Balance the following equation: Bi(OH)3 +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents