In the structure below, the hybridization of the oxygen is ________ and the C-O-C bond angle is ________. 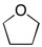
A) sp2; 120°
B) sp2; <109.5°
C) sp3; 120°
D) sp3; <109.5°
E) sp; 120°
Correct Answer:
Verified
Q18: Which of the following statements about π
Q19: Which atomic orbital combination would result in
Q20: What kind of molecular orbital (σ, σ*,
Q21: What two hybrid atomic orbitals overlap to
Q22: Shown below is one of the sex
Q24: Draw a three-dimensional model of methanol, CH3OH,
Q25: Choose the term below which best describes
Q26: Choose the correct hybridization for the atom
Q27: In the structure below, the sigma bond
Q28: What is the approximate value of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents