Structures ________, shown below, are resonance structures, and structure ________ is the major contributor to the overall resonance hybrid. 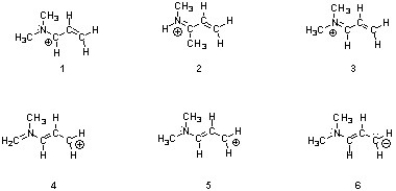
A) 2 & 4; 2
B) 1, 3 & 5; 3
C) 4 & 6; 6
D) 1, 3 & 5; 1
E) 1, 3, 4 & 5; 3
Correct Answer:
Verified
Q40: Write a Lewis structure for a compound
Q41: The Lewis structure of trimethylamine is shown
Q42: Nitroamines are common functional groups found in
Q43: Which of the following compounds are covalent
Q44: When a negatively charged species is most
Q46: Draw the important resonance forms for the
Q47: In the compound sodium methoxide (NaOCH3), there
Q48: Draw the complete Lewis structure for the
Q49: One resonance structure of a cation is
Q50: Draw the other important resonance form of:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents