Nitroamines are common functional groups found in energetic materials, such as RDX and HMX. For the structure below, draw two other significant resonance structures, include any formal charges, and indicate the hybridization on each nitrogen and oxygen. 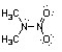
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q37: Draw the Lewis structure for boric acid,
Q38: Within a given row of the periodic
Q39: The compound methylamine, CH3NH2, contains a C-N
Q40: Write a Lewis structure for a compound
Q41: The Lewis structure of trimethylamine is shown
Q43: Which of the following compounds are covalent
Q44: When a negatively charged species is most
Q45: Structures _, shown below, are resonance structures,
Q46: Draw the important resonance forms for the
Q47: In the compound sodium methoxide (NaOCH3), there
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents