Which of the following best explains why the synthetic route shown below would be unsuccessful? 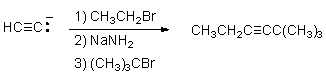
A) The alkynide anion is not a strong enough nucleophile to react with 1-bromopropane in step 1.
B) Sodium amide is not a strong enough base to deprotonate the terminal alkyne in step 2.
C) The alkynide anion formed by reaction with sodium amide will facilitate an E2 (rather than SN2) reaction with t-butyl bromide.
D) Both substitution reactions will occur on the same end of the alkyne,making a product different than the one shown.
E) Reaction with sodium amide will result in formation of a primary alkyne.
Correct Answer:
Verified
Q151: Which sequence of reactions is expected to
Q152: Provide the structure(s)of the expected major organic
Q153: Provide the reagent(s)expected to accomplish the transformation
Q154: Which sequence of reactions is expected to
Q155: Provide the systematic IUPAC name for CH3CHBrC≡C(CH2)3CH3.
Q156: Which sequence of reactions is expected to
Q157: Provide the structure(s)of the expected major organic
Q158: Provide the structure(s)of the expected major organic
Q159: Provide the systematic IUPAC name for CH3C≡CC(CH3)2CH(CH2CH3)2.
Q160: Provide the systematic IUPAC name for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents