Why is the following not a good two step route for the preparation of pure L-alanine? 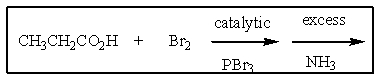
A) The bromination is not selective (the CH3 and CH2 groups are both brominated) .
B) Ammonia is not sufficiently nucleophillic to react with a secondary bromide.
C) Only the CH3 group is brominated.
D) The product will be racemic.
E) Propanamide would be the major product.
Correct Answer:
Verified
Q1: Which of the following is least likely
Q2: Which of the following amino acids would
Q4: Disulfide bonds in proteins
A) result from oxidation
Q5: Why is the additional (ring)nitrogen in tryptophan
Q6: The primary structure of a protein refers
Q7: What material can be used repetitively (on
Q8: Nearly all amino acids immediately release N2
Q9: Which of the following amino acids is
Q10: What is dicyclohexylcarbodiimide (DCC)used for in peptide
Q11: What is the name of the DNA
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents