A sample of an ideal gas is slowly compressed to one-half its original volume with no change in pressure. If the original root-mean-square speed (thermal speed) of the gas molecules was V, the new speed is
A) V.
B) 2V.
C) 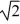 V.
V.
D) V/2.
E) V/ 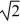
Correct Answer:
Verified
Q1: The average molecular kinetic energy of a
Q4: An ideal gas is kept in a
Q11: The second law of thermodynamics leads us
Q15: The entropy of an isolated system must
Q21: The mean free path of an oxygen
Q27: A 5.0-liter gas tank holds 1.7 moles
Q28: An oxygen molecule falls in a vacuum.From
Q29: The root-mean-square speed (thermal speed)of a certain
Q35: A sealed container holds 0.020 moles of
Q42: Eleven molecules have speeds 16,17,18,...,26 m/s.Calculate the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents