If we double the root-mean-square speed (thermal speed) of the molecules of a gas, then
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of 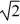 .
.
D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.
Correct Answer:
Verified
Q4: What is the total translational kinetic energy
Q7: If the temperature of a fixed amount
Q7: What is the average translational kinetic energy
Q10: As a result of any natural process,the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents