A 0.10- 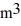 gas tank holds 5.0 moles of nitrogen gas (N2) , at a temperature of 370 K. The atomic mass of nitrogen is 14 g/mol, the molecular radius is 3.0 × 10-10 m, and the Boltzmann constant is 1.38 × 10-23 J/K. The root-mean-square speed (thermal speed) of the molecules is closest to
gas tank holds 5.0 moles of nitrogen gas (N2) , at a temperature of 370 K. The atomic mass of nitrogen is 14 g/mol, the molecular radius is 3.0 × 10-10 m, and the Boltzmann constant is 1.38 × 10-23 J/K. The root-mean-square speed (thermal speed) of the molecules is closest to
A) 570 m/s.
B) 810 m/s.
C) 410 m/s.
D) 22 m/s.
E) 99 m/s.
Correct Answer:
Verified
Q5: A mole of oxygen (O2)molecules and a
Q6: If we double the root-mean-square speed (thermal
Q7: What is the average translational kinetic energy
Q13: A container is filled with a mixture
Q15: The entropy of an isolated system must
Q16: A 5.0-liter gas tank holds 1.4 moles
Q17: According to the second law of thermodynamics,the
Q22: The root-mean-square speed (thermal speed)for a certain
Q23: An ideal gas is kept in a
Q27: A 5.0-liter gas tank holds 1.7 moles
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents