Exhibit 6-9
Use the reaction energy diagram below to answer the following question(s) .
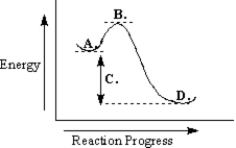
-Refer to Exhibit 6-9.The reaction depicted in this reaction energy diagram can best be described as:
A) a slow exothermic reaction
B) a fast exothermic reaction
C) a slow endothermic reaction
D) a fast endothermic reaction
Correct Answer:
Verified
Q14: _ ΔG° = −RT ln Keq
A)transition state
B)endergonic
Q15: Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with
Q16: Exhibit 6-9
Use the reaction energy diagram below
Q17: _ The energy needed by reactants to
Q18: _ A species that lies at an
Q20: Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with
Q21: The alkane formed by hydrogenation of (S)-4-methyl-1-hexene
Q22: Consider the following process. Q23: Which of the following could act as Q24: Which of the following could act as![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents