Consider the following process. 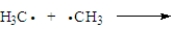 Which of the following correctly applies?
Which of the following correctly applies?
A) termination step
B) unsymmetrical bond formation
C) polar reaction
D) produces a radical
Correct Answer:
Verified
Q17: _ The energy needed by reactants to
Q18: _ A species that lies at an
Q19: Exhibit 6-9
Use the reaction energy diagram below
Q20: Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with
Q21: The alkane formed by hydrogenation of (S)-4-methyl-1-hexene
Q23: Which of the following could act as
Q24: Which of the following could act as
Q25: Consider the following grayscale electrostatic potential map.The
Q26: Write the mechanism of the reaction of
Q27: Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents