At atmospheric pressure,45 moles of liquid helium are vaporized at its boiling point of 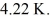 The heat of vaporization of helium,at atmospheric pressure,is
The heat of vaporization of helium,at atmospheric pressure,is 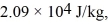 and the atomic weight of helium is
and the atomic weight of helium is 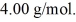 The change in the entropy of the helium,as it vaporizes,is closest to
The change in the entropy of the helium,as it vaporizes,is closest to
A) 890 J/K.
B) 14,000 J/K.
C) 18,000 J/K.
D) -9400 J/K.
E) -14,000 J/K.
Correct Answer:
Verified
Q27: A Carnot refrigerator takes heat from water
Q29: A perfect Carnot engine operates between the
Q32: A Carnot refrigerator has a coefficient of
Q40: A Carnot engine operates between reservoirs at
Q40: The temperature inside a Carnot refrigerator placed
Q41: A 810-g quantity of ethanol,in the liquid
Q41: A 2.0-kg block of aluminum at 50°C
Q42: A brass rod,75.0 cm long and having
Q43: A 2.00-kg block of ice at 0.00°C
Q47: A 2.00 kg piece of lead at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents