Assuming the radius of diatomic molecules is approximately 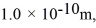 for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K,Avogadro's number is 6.02 × 1023 molecules/mole,and the ideal gas constant is
for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K,Avogadro's number is 6.02 × 1023 molecules/mole,and the ideal gas constant is 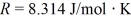 = 0.0821 L ∙ atm/mol ∙ K.
= 0.0821 L ∙ atm/mol ∙ K.
A) 4.9 × 10-8 atm
B) 6.9 × 10-8 atm
C) 1.5 × 10-7 atm
D) 2.2 × 10-7 atm
Correct Answer:
Verified
Q11: The second law of thermodynamics leads us
Q13: A container is filled with a mixture
Q19: A container is filled with a mixture
Q21: The mean free path of an oxygen
Q23: Dust particles are pulverized rock,which has density
Q24: What is the average kinetic energy of
Q27: A 5.0-liter gas tank holds 1.7 moles
Q27: The mean free path of an oxygen
Q29: The root-mean-square speed (thermal speed)of a certain
Q48: At 50.0°C,the average translational kinetic energy of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents