If you add 700 kJ of heat to 700 g of water at 70.0°C,how much water is left in the container? The latent heat of vaporization of water is 2.26 × 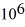 J/kg and its specific heat is 4190 J/(kg ∙ K) .
J/kg and its specific heat is 4190 J/(kg ∙ K) .
A) 429 g
B) 258 g
C) 340 g
D) 600 g
E) none
Correct Answer:
Verified
Q30: A block of ice at 0.000°C is
Q37: A person makes ice tea by adding
Q38: A 200-g metal container,insulated on the outside,holds
Q39: A substance has a melting point of
Q42: The filament in a light bulb has
Q43: The figure shows a pV diagram for
Q45: A rod,with sides insulated to prevent heat
Q50: Under steady state conditions,a piece of wood
Q54: A cube at 100°C radiates heat at
Q63: What is the net power that a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents