The figure shows a pV diagram for 4.3 g of oxygen gas (O2) in a sealed container.The temperature T1 of the gas in state 1 is 21°C.What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K,and the ATOMIC weight of oxygen is 16 g/mol. 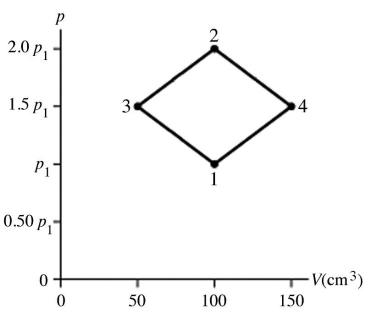
A) -52°C,390°C
B) 16°C,47°C
C) 220°C,660°C
D) 11°C,32°C
Correct Answer:
Verified
Q38: A 200-g metal container,insulated on the outside,holds
Q39: A substance has a melting point of
Q40: If you add 700 kJ of heat
Q42: The filament in a light bulb has
Q45: A rod,with sides insulated to prevent heat
Q46: A solid metal sphere is 15.0 cm
Q48: The figure shows a pV diagram for
Q51: A concrete wall of a cold storage
Q54: A cube at 100°C radiates heat at
Q63: What is the net power that a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents