The figure shows the pV diagram for a certain thermodynamic process.In this process,
1500 J of heat flows into a system,and at the same time the system expands against a constant external pressure of 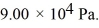 If the volume of the system increases from
If the volume of the system increases from 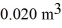 to
to 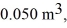 calculate the change in internal (thermal)energy of the system.If the internal (thermal)energy change is nonzero,be sure to indicate whether this energy change is positive or negative.
calculate the change in internal (thermal)energy of the system.If the internal (thermal)energy change is nonzero,be sure to indicate whether this energy change is positive or negative. 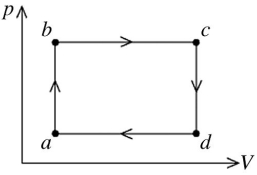
Correct Answer:
Verified
Q39: In an isochoric process,the internal (thermal)energy of
Q47: An ideal gas initially at 300 K
Q49: A cylinder contains 23 moles of an
Q50: An ideal gas is allowed to expand
Q51: An ideal gas with γ = 1.30
Q51: A cylinder contains 24.0 moles of an
Q87: The gas in a perfectly insulated system
Q88: A fixed amount of ideal gas goes
Q92: A container with rigid walls is filled
Q95: In a thermodynamic process involving 7.8 moles
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents