A fixed amount of ideal gas goes through a process abc.In state a,the temperature of the gas is 152°C,its pressure is 1.25 atm,and it occupies a volume of 0.250 m3.It then undergoes an isothermal expansion to state b that doubles its volume,followed by an isobaric compression back to its original volume at state c.(Hint: First show this process on a pV diagram.)The ideal gas constant is 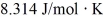 ,and 1.00 atm = 1.01 × 105 Pa.
,and 1.00 atm = 1.01 × 105 Pa.
(a)How many moles does this gas contain?
(b)What is the change in the internal energy of the gas between states a and b?
(c)What is the net work done on (or by)this gas during the entire process?
(d)What is the temperature of the gas in state c?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q25: A system has a heat source supplying
Q27: In an isochoric process,the internal (thermal)energy of
Q39: In an isochoric process,the internal (thermal)energy of
Q45: During an adiabatic process, 20 moles of
Q47: An ideal gas initially at 300 K
Q49: A cylinder contains 23 moles of an
Q51: An ideal gas with γ = 1.30
Q87: The gas in a perfectly insulated system
Q92: A container with rigid walls is filled
Q93: The figure shows the pV diagram for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents