The G'° values for the two reactions shown below are given.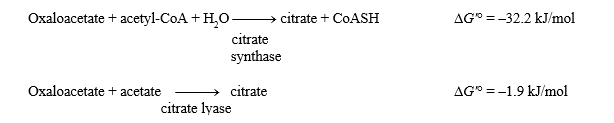
What is the G'° for the hydrolysis of acetyl-CoA?
Acetyl-CoA + H2O acetate + CoASH + H+
A) -34.1 kJ/mol
B) -32.2 kJ/mol
C) -30.3 kJ/mol
D) +61.9 kJ/mol
E) +34.1 kJ/mol
Correct Answer:
Verified
Q2: For the reaction A
Q4: Which of the following is true about
Q5: When a mixture of glucose 6-phosphate
Q6: During glycolysis,glucose 1-phosphate is converted to
Q7: Which of the following is not electrophilic?
A)A
Q8: Which of the following compounds has
Q9: Which of the following is
Q10: Which of the following is not nucleophilic?
A)A
Q11: For the reaction A
Q18: For the following reaction,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents