How many ð electron pairs are present in the following aromatic heterocycle? 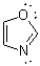
A) 2
B) 3
C) 4
D) 5
Correct Answer:
Verified
Q33: How many ð electron pairs are in
Q34: Increased conjugation leads to an increase in
Q35: In the aromatic compound resonance form shown
Q36: How many electrons pairs are part of
Q37: Which of the following best describes anti-aromatic
Q39: Which of the following best describes the
Q40: The bond length of a conjugated double
Q41: The nitrogen atom in amide bonds is
Q42: Assuming planarity,the following ion is considered aromatic.
Q43: Lone pairs cannot be a part of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents