True/False
Assuming planarity,the following ion is considered aromatic. 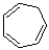
Correct Answer:
Verified
Related Questions
Q37: Which of the following best describes anti-aromatic
Q38: How many ð electron pairs are present
Q39: Which of the following best describes the
Q40: The bond length of a conjugated double
Q41: The nitrogen atom in amide bonds is
Q43: Lone pairs cannot be a part of
Q44: Alkynes cannot be a part of conjugated
Q45: Sigma bonds in conjugated systems have a
Q46: The larger the difference in energy between
Q47: Anti-aromatic compounds can be non-planar.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents