Assuming planarity,the following molecule is considered aromatic. 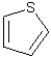
Correct Answer:
Verified
Q51: Assuming planarity,the following ion is considered anti-aromatic.
Q52: All lone pair electrons are considered ð
Q53: All planar molecules are considered aromatic.
Q54: A molecule must be planar to be
Q55: Carbanions are anti-aromatic,whereas carbocations are aromatic.
Q57: Amide bonds undergo free rotation at room
Q58: Only ð electrons contribute towards aromaticity.
Q59: Anti-aromatic compounds are also considered non-aromatic.
Q60: Assuming planarity,the following molecule is considered non-aromatic.
Q61: Benzene has a _ heat of hydrogenation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents