Assuming planarity,the following ion is considered anti-aromatic. 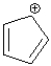
Correct Answer:
Verified
Q46: The larger the difference in energy between
Q47: Anti-aromatic compounds can be non-planar.
Q48: Given the option,molecular structures tend to exist
Q49: Energy of light is inversely proportional to
Q50: Any molecule that follows Hückel's rule is
Q52: All lone pair electrons are considered ð
Q53: All planar molecules are considered aromatic.
Q54: A molecule must be planar to be
Q55: Carbanions are anti-aromatic,whereas carbocations are aromatic.
Q56: Assuming planarity,the following molecule is considered aromatic.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents