Which acid shown below is stronger and why? 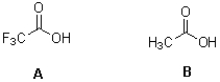
A) A due to charge delocalization
B) B due to charge delocalization
C) A due to induction
D) B due to induction
Correct Answer:
Verified
Q2: Figure 1
Figure 1 is a list of
Q3: Given the structure and pKa of analine
Q4: What is the definition of a Lewis
Q5: What is the definition of a Brønsted
Q6: Given the structure and pKa of 2,2,2
Q8: Which acid shown below is stronger and
Q9: Which acid shown below is stronger and
Q10: What is the role of induction in
Q11: Given the structure and pKa of pyridine
Q12: Which side will the equilibrium of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents