Given the structure and pKa of analine (shown below) ,what can be said about its protonation state at pH 4.9? 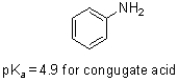
A) ˜100% 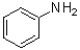
B) ˜100% 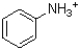
C) ˜50% 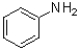 ,˜50%
,˜50%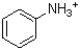
D) There is not enough data to evaluate.
Correct Answer:
Verified
Q1: What hybridization best stabilizes the negative charge
Q2: Figure 1
Figure 1 is a list of
Q4: What is the definition of a Lewis
Q5: What is the definition of a Brønsted
Q6: Given the structure and pKa of 2,2,2
Q7: Which acid shown below is stronger and
Q8: Which acid shown below is stronger and
Q9: Which acid shown below is stronger and
Q10: What is the role of induction in
Q11: Given the structure and pKa of pyridine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents