Which acid shown below is stronger and why? 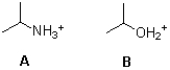
A) A because nitrogen is more electronegative
B) A because oxygen is more electronegative
C) B because nitrogen is more electronegative
D) B because oxygen is more electronegative
Correct Answer:
Verified
Q11: Given the structure and pKa of pyridine
Q12: Which side will the equilibrium of the
Q13: What is the role of atomic size
Q14: Which of the following best describes BF3?
A)Brønsted
Q15: What characterizes weak acids in comparison to
Q17: Which is the most acidic proton in
Q18: Which of the phenols shown below is
Q19: Rank these compounds in order of increasing
Q20: Figure 1
Figure 1 is a list of
Q21: Which molecule shown below would you expect
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents