Which molecule shown below would you expect to be most acidic and why? 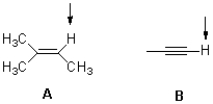
A) A,due to charge delocalization
B) B,due to charge delocalization
C) A,due to hybridization
D) B,due to hybridization
Correct Answer:
Verified
Q16: Which acid shown below is stronger and
Q17: Which is the most acidic proton in
Q18: Which of the phenols shown below is
Q19: Rank these compounds in order of increasing
Q20: Figure 1
Figure 1 is a list of
Q22: Given the following reaction scheme,what best describes
Q23: Electron withdrawing groups stabilize conjugate bases.
Q24: Which of the following best describes the
Q25: Rank the following molecules in terms of
Q26: Given the following reaction scheme,what best describes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents