What is the strongest intermolecular force that the molecule shown below is capable of making? 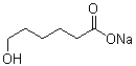
A) London dispersion
B) electrostatics
C) dipole-dipole
D) hydrogen bonding
Correct Answer:
Verified
Q24: Which of the following best describes the
Q25: How many hydrogen bond donors does the
Q26: Which of the following best describes the
Q27: How many hydrogen bond accepting pairs of
Q28: Which functional group is NOT present in
Q30: Rank the following in terms of increasing
Q31: Which statement best describes the two molecules
Q32: How many ð bonds can be found
Q33: Which of the following best describes the
Q34: Which functional group is present in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents