Identify the electron pair geometry and hybridization of every carbon atom in the following structure.Draw a resonance structure and the resonance hybrid of the following structure. 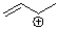
Correct Answer:
Verified
Carbon - Trigonal pl...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q74: Resonance requires atoms with neighbouring aligned p
Q75: Electrons shared among atoms are said to
Q76: An sp2 hybridized carbon has an orbital
Q77: A carbocation with three ó bonds has
Q78: A carbon atom with two ð bonds
Q79: An sp hybridized atom has a _
Q80: The combined form of all resonance structures
Q81: Draw the following molecule in zig-zag format:
Q82: Assign the electron pair geometry and hybridization
Q83: How many atoms share delocalized orbitals with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents