The temperature at which the following process reaches equilibrium at 1.0 atm is the normal melting point for phosphoric acid. H3PO4(s)  H3PO4(l)
H3PO4(l)
Use the following thermodynamic information at 298 K to determine this temperature.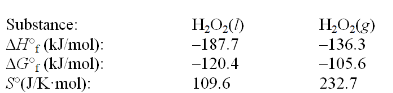
A) 286 K
B) 305 K
C) 315 K
D) 347 K
E) 3170 K
Correct Answer:
Verified
Q41: For a process with ΔS < 0,
Q45: Calculate ΔG° for the combustion of propane.
Q45: Given: H2O(l) → H2O(s) ΔH° = −6.02
Q46: Nitric oxide reacts with chlorine to form
Q48: Calculate ΔG° for the reaction of ammonia
Q52: Which of the following conditions will ensure
Q54: The second law of thermodynamics tells us
Q54: Consider the figure that shows ΔG° for
Q56: A certain process has ΔH° > 0,
Q57: In order for a process to be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents